Postdoctoral Research at MGH
Positron emission tomography (PET) enables 3D visualization of vital physiological information, e.g., metabolism, blood flow, and neuroreceptor concentration by using targeted radioisotope-labeled tracers. Quantitative interpretation of PET images is crucial both in diagnostic and therapeutic contexts in many areas of medicine, including oncology, neurology, and cardiology. My postdoctoral research at Massachusetts General Hospital (MGH) under the mentorship of Drs. Quanzheng Li and Georges El Fakhri involved developing a range of techniques for motion correction, denoising, and deblurring of PET images with the goal of enhancing the quantitative capabilities of PET. Several of these methods achieve this by incorporating information from an anatomical imaging modality such as magnetic resonance imaging (MRI). Some of these projects are described below.
My postdoctoral research highlighted on the covers of the journals Medical Physics and Theranostics.
Motion-Compensated Image Reconstruction
Simultaneous PET/MRI combines the strengths of two complementary imaging modalities and is a potent tool for integrated imaging. While PET reveals only functional or physiological information, MRI (magnetic resonance imaging) is able to generate structural or anatomical information, generally with higher resolution. In the context of lung imaging, where PET scans are severely compromised by respiratory motion, we have developed a maximum a posteriori estimation framework that incorporates deformation fields derived from simultaneously acquired MRI data. This technique enables the generation of PET images free of motion artifacts, which leads to improved image quantitation, thereby facilitating lung cancer staging and treatment optimization.
Links: Med Phys 2015 Paper, IEEE MIC 2012 Paper
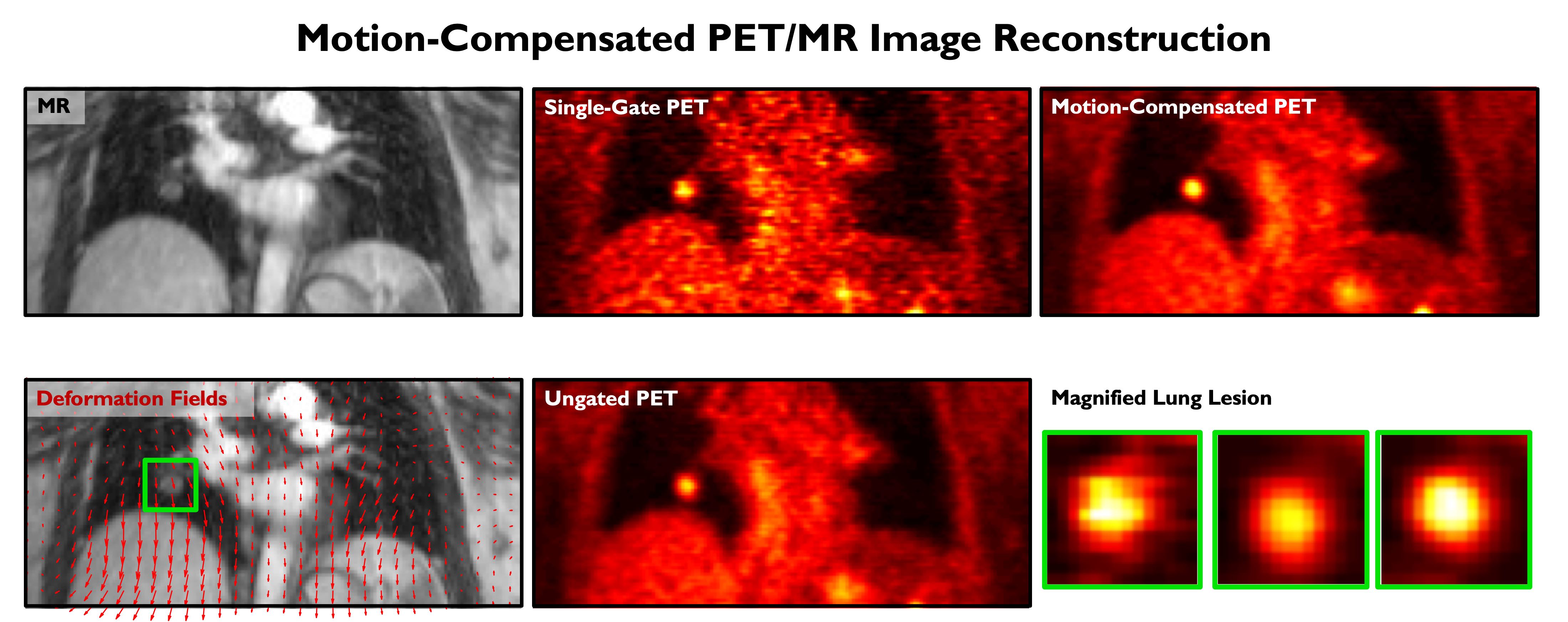
An MR image, computed deformation due to breathing motion (indicated by red arrows overlaid on the MR), a single-gate PET image (noisy with less motion artifacts), an ungated PET image (with low noise but with motion-induced blurring), a motion-compensated PET image (with low noise and less motion artifacts), and a magnified lung lesion from all three PET images.
Image Denoising
The high levels of statistical noise in PET images pose a challenge to accurate quantitation. This issue is particularly well-pronounced at the early time frames of dynamic PET images, which are usually short to capture rapid changes in tracer uptake patterns. We developed a non-local means denoising filter for dynamic PET images which uses spatiotemporal patches for robust similarity computation. Realistic simulations of a dynamic digital mouse phantom showed improved bias-variance performance characterics relative to several well-known denoising approaches. Experiments in mice and humans showed clear improvement in contrast-to-noise ratio in Patlak parametric images.
Link: PLOS One 2013 Paper
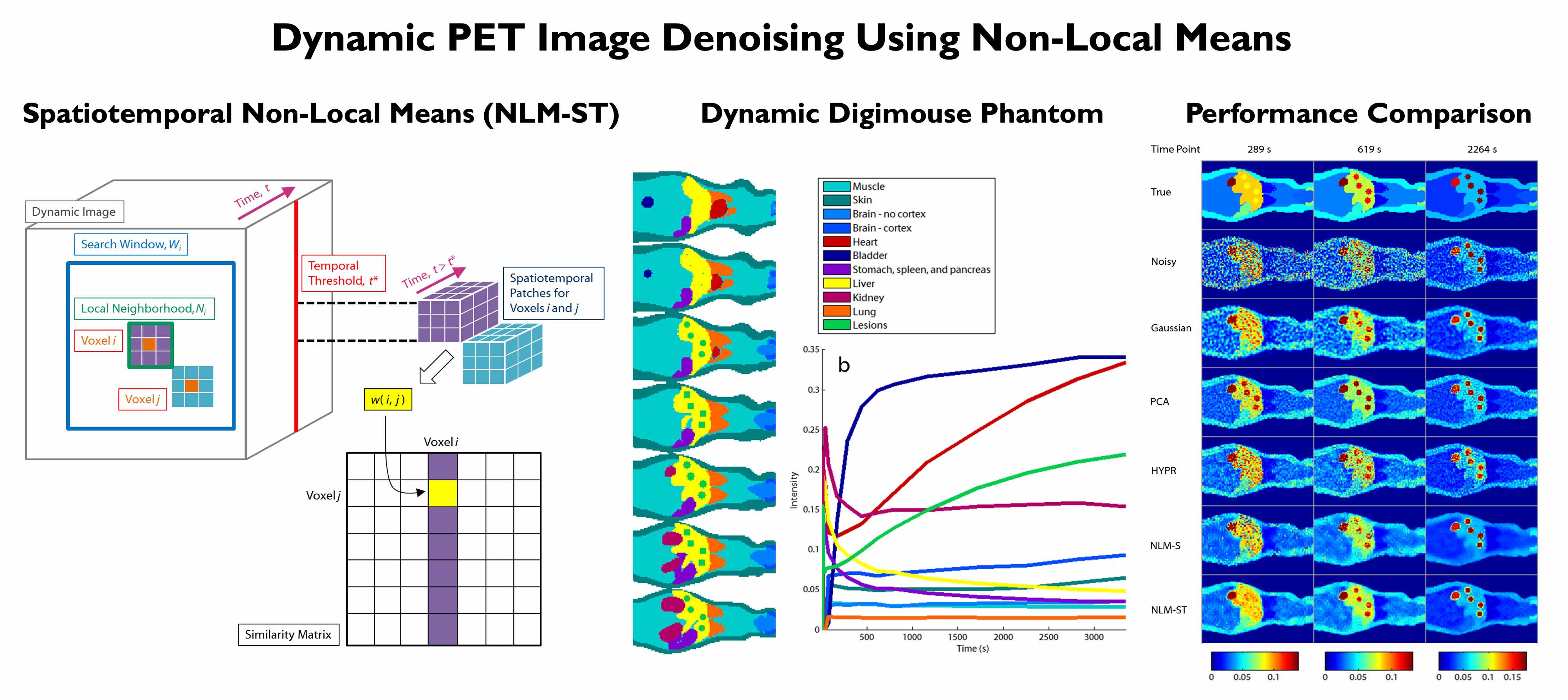
[From left to right] Computation of non-local similarities from spatiotemporal image patches extracted from dynamic PET images. A dynamic Digimouse phantom created for simulation studies. Noisy and denoised images for comparison of spatiotemporal non-local means (NLM-ST) with alternative denoising strategies.
Image Deblurring
The quantitative accuracy of PET is degraded by partial volume effects caused by the limited spatial resolution capabilities of PET scanners. We developed a method for PET image deblurring that exploits the higher resolution imaging capabilities of high-resolution anatomical magnetic resonance (MR) images. This method performs spatially variant deconvolution with a joint entropy (JE) penalty function. One of the target applications for this project is Alzheimer's disease (AD), which is a debilitating neurodegenerative disorder that affects over 30 million people worldwide. The hallmark pathologies of AD are histology-confirmed amyloid-β (Aβ) plaques and tau neurofibrillary tangles, both of which appear many years before the onset of cognitive decline. Application of our deblurring method to tau images using Flortaucipir PET on cognitively normal and impaired subjects revealed that deblurring leads to a marked improvement in the correlation of PET measures with well-recognized clinical metrics of cognitive performance.
Links: ISBI 2015 Paper, IEEE TCI 2019 Paper
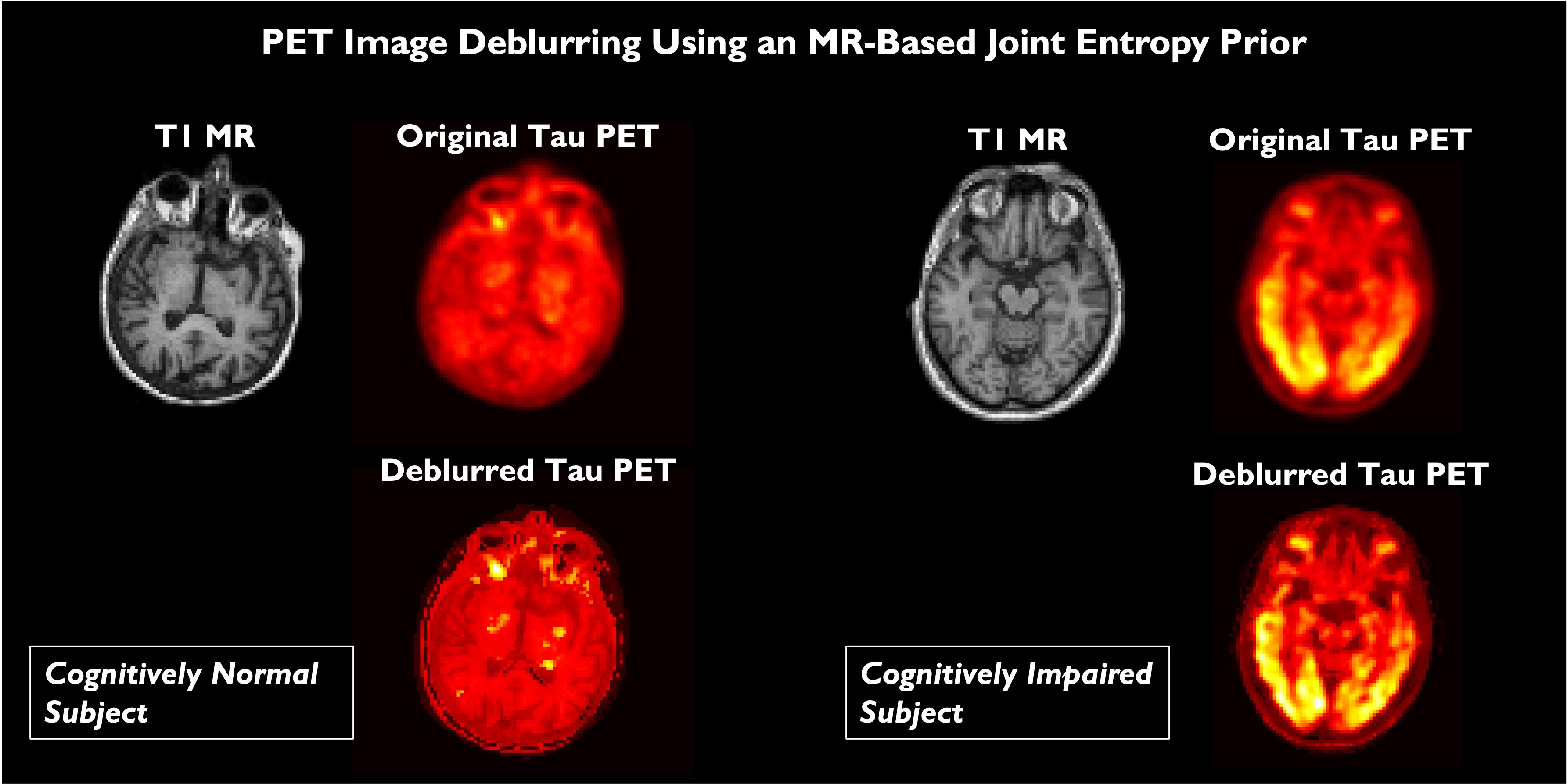
Comparison of brain scans of a cognitively normal subject and and Alzheimer's disease patient. The subfigures show A. T1-weighted MPRAGE MRI images, B. Flortaucipir PET images of tau tangles reconstructed by the scanner, C.Flortaucipir PET images deblurred with a measured spatially varying point spread function, and D. Flortaucipir PET images deblurred with a measured spatially varying point spread function with the help of a MRI-based joint entropy prior.
Brain Network Analysis
Aβ and tau protein aggregates, which are hallmarks of AD, have characteristic spatial patterns with links to disease progression. Brain network connectivity studies are important for revealing the spatial associations in these patterns. It is well-known now that neurological disorders like AD disrupt both structural and functional connectivity of brain networks. We have developed techniques based on graph theory and graph signal processing to interpret and analyze brain connectivity. We were able to demonstrate that PET image deblurring using the JE-based method that we developed leads to increased discriminative power in the brain network domain for functional brain networks generated from Aβ PET imaging.
Links: Sci Rep 2017 Paper
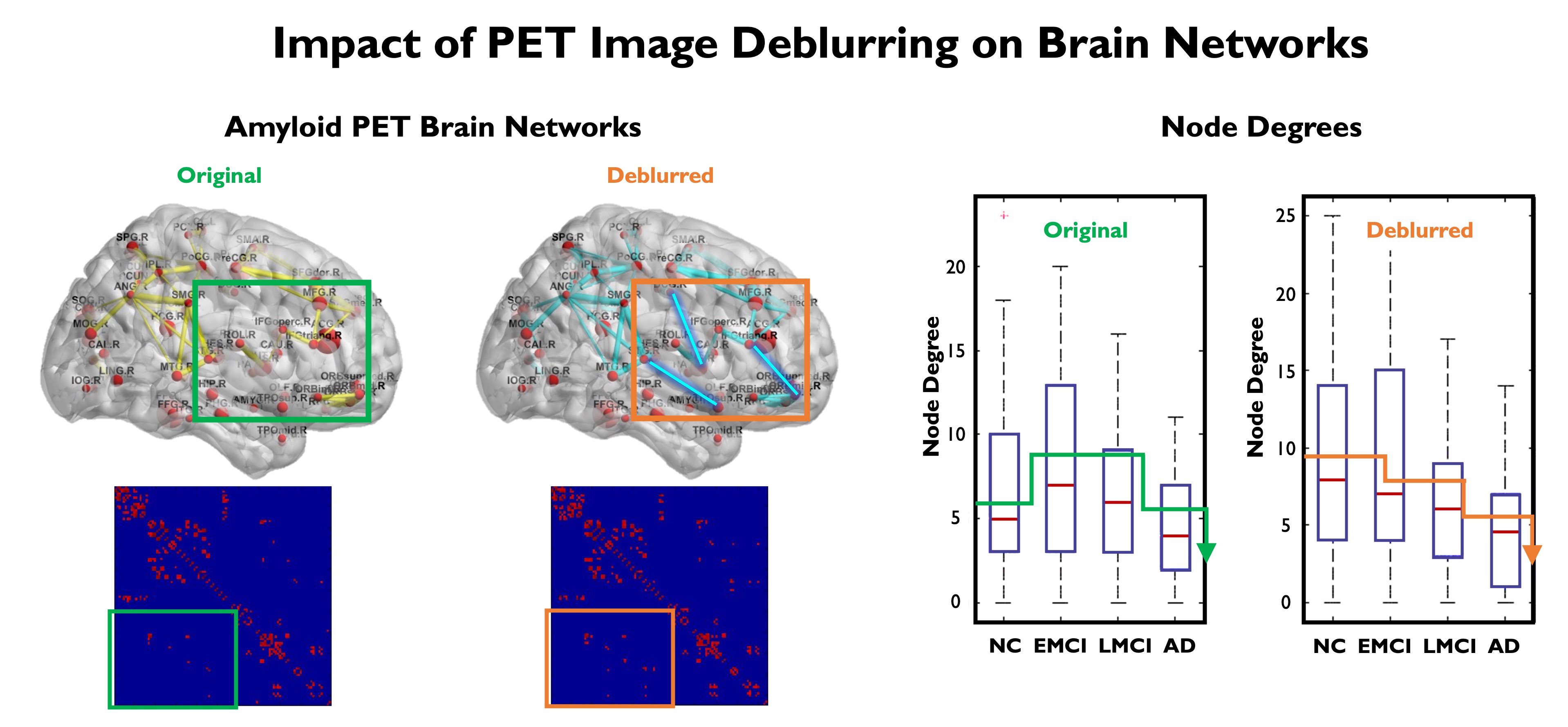
Comparison of Aβ networks obtained from original (uncorrected) and deblurred (partial volume corrected) Florbetabir datasets from ADNI. The subfigures show A. Brain network and graph adjacency matrix obtain from original Florbetabir images, B. FBrain network and graph adjacency matrix obtain from deblurred Florbetabir images showing recovery of previously unseen inter-regional connections (edges) in the graph, C. Node degrees for four populations: normal controls (NC), early mild cognitive impairment (EMCI), late mild cognitive impairment (LMCI), and Alzheimer's disease (AD). Deblurring of Florbetabir images led to a consistently decreasing trend in the observed node degree with disease progression.